Gleevec is under patent in the U.S., but not in India. Accordingly, Novartis, its Swiss-based manufacturer, may prevent competitors from making and selling lower-cost versions of the drug in the U.S., but not in India.
Rejecting the Gleevec patent application is not the only step that the Indian government has taken to circumvent patents on cancer drugs. Last year, India issued a compulsory license on Nexavar, a late-stage kidney and liver cancer treatment, enabling a local drug firm to produce a generic version of this medicine without the permission of Bayer, the patent holder. India has recently announced plans to grant compulsory licenses on another leukemia drug and two breast cancer therapies.
What is Gleevec?

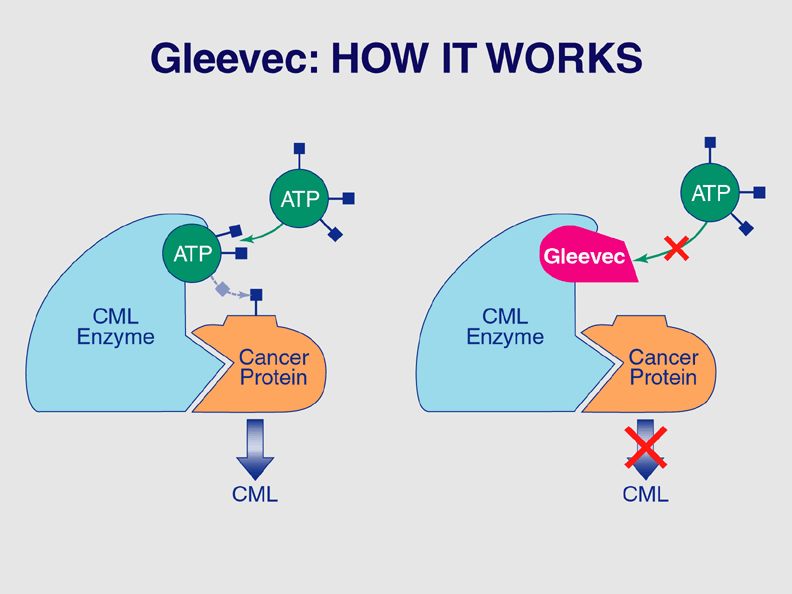
Gleevec (imatinib) interferes with the growth of some cancer cells.
Gleevec is used to treat certain types of leukemia (blood cancer), bone marrow disorders, and skin cancer, or certain tumors of the stomach and digestive system.
Gleevec may also be used for purposes not listed in this medication guide.
Important information
Gleevec can lower blood cells that help your body fight infections and help your blood to clot. You may get an infection or bleed more easily. Call your doctor if you have unusual bruising or bleeding, or signs of infection (fever, chills, body aches).
Gleevec may increase the risk of fluid retention or heart problems. Tell your doctor if you have swelling or rapid weight gain, shortness of breath, fast or slow heart rate, weak pulse, or fainting.
Gleevec side effects
Get emergency medical help if you have any of these signs of an allergic reaction to Gleevec: hives; difficulty breathing; swelling of your face, lips, tongue, or throat.
Call your doctor at once if you have:
- fever, chills, body aches, flu symptoms;
- easy bruising, unusual bleeding (nose, mouth, vagina, or rectum), purple or red pinpoint spots under your skin;
- swelling, rapid weight gain, feeling short of breath
 (even with mild exertion);
(even with mild exertion); - nausea, upper stomach pain, itching, loss of appetite, dark urine, clay-colored stools, jaundice (yellowing of the skin or eyes);
- bloody or tarry stools, coughing up blood or vomit that looks like coffee grounds;
- lower back pain
 , blood in your urine, little or no urinating;
, blood in your urine, little or no urinating; - numbness or tingly feeling around your mouth;
- muscle weakness, tightness, or contraction, overactive reflexes;
- fast or slow heart rate, weak pulse, confusion, fainting; or
- severe skin reaction — fever, sore throat, swelling in your face or tongue, burning in your eyes, skin pain, followed by a red or purple skin rash that spreads (especially in the face or upper body) and causes blistering and peeling.
Common Gleevec side effects![]() may include:
may include:
- mild nausea or stomach pain, vomiting, diarrhea;
- joint or muscle pain; or
- headache, feeling tired.
This is not a complete list of side effects and others may occur. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
What other drugs will affect Gleevec?
Many drugs can interact with Gleevec. Not all possible interactions are listed here. Tell your doctor about all your medications and any you start or stop using during treatment with Gleevec, especially:
- acetaminophen (Tylenol);
- aprepitant;
- bosentan;
- cimetidine;
- cyclosporine;
- haloperidol;
- St. John’s wort;
- an antibiotic–clarithromycin, ciprofloxacin, doxycycline, erythromycin, metronidazole, nafcillin, norfloxacin, rifabutin, rifampin, rifapentine, tetracycline;
- antifungal medication–clotrimazole, fluconazole, itraconazole, ketoconazole, posaconazole, voriconazole;
- an antidepressant–desipramine, nefazodone, sertraline;
- a blood thinner such as warfarin, Coumadin;
- heart or blood pressure medication–amiodarone, diltiazem, dronedarone, lidocaine, nicardipine, quinidine, verapamil;
- hepatitis C medications–boceprevir, telaprevir;
- HIV/AIDS medication–atazanavir, delavirdine, darunavir when given with ritonavir, efavirenz, fosamprenavir, indinavir, nelfinavir, nevirapine, ritonavir, saquinavir; or
- seizure medication–carbamazepine, fosphenytoin, oxcarbazepine, phenobarbital, phenytoin, primidone.
This list is not complete and many other drugs can interact with Gleevec. This includes prescription and over-the-counter medicines, vitamins, and herbal products. Give a list of all your medicines to any healthcare provider who treats you.
FDA Approves Important New Leukemia Drug
Offers Further Proof of Principle for Molecular Targeting in Cancer Treatment
The U.S. Food and Drug Administration (FDA) announced today its approval of the drug Gleevec as an oral treatment for chronic myelogenous leukemia (CML).
The announcement, issued at a press conference in Washington, also marks the approval of the first drug that directly turns off the signal of a protein known to cause a cancer. Other molecular-targeting drugs previously approved by the FDA interfere with proteins associated with other cancers, but not with proteins that directly cause the disease.
Gleevec, also known as STI571, was discovered and developed by Novartis Oncology in collaboration with its colleagues in academia.
CML is a disease in which too many white blood cells are made in the bone marrow, the spongy tissue inside the large bones in the body. Most of the 4,500 Americans diagnosed with CML each year are middle-aged or older, although the cancer can occur in children. In the first stages of CML, most people do not have any symptoms of cancer, and the disease progresses slowly.
Bone marrow transplantation in the initial chronic phase of the disease is the only known cure for CML. However, many patients are not young or healthy enough to tolerate transplantation or do not have a suitable marrow donor, and the procedure can cause serious side effects or death.
Treatment with the drug interferon alfa may produce remission, restoring a normal blood count in up to 70 percent of patients with chronic phase CML. If interferon alfa is ineffective or patients stop responding to the drug, the prognosis is generally bleak.
Gleevec has produced higher remission rates in three short-duration, early phase clinical trials. In the results of one clinical trial, reported in April in the New England Journal of Medicine, Gleevec restored normal blood counts in 53 out of 54 interferon-resistant CML patients, a response rate rarely seen in cancer with a single agent. Fifty-one of these patients were still doing well after a year on the medicine, and most reported few minor side effects.
In March 2001, the FDA granted an expedited review of Gleevec based on these clinical trials. Today, after only two months of review–one of the fastest reviews ever for a cancer drug –the FDA approved Gleevec.
Richard Klausner, M.D., director of the National Cancer Institute, said that he shared in the excitement of people with CML, but he also mentioned several caveats. He said many research questions remain to be answered, such as how long Gleevec will control CML. Does the drug actually cure CML patients? Or, does it delay the onset of more advanced forms of the cancer?
Klausner said NCI, in a cooperative research and development agreement (CRADA) with Novartis Oncology, is now investigating these issues in ongoing clinical trials involving people with CML. Several clinical trials are also testing the possible effectiveness of Gleevec in other cancers including glioma, soft tissue sarcoma, and a rare kind of gastrointestinal cancer called gastrointestinal stromal tumor (GIST).
Despite the caveats, Klausner said Gleevec serves as a bellwether for other molecular-targeting cancer drugs. He said an intensive effort is now under way in academia and industry to identify unique, cancer-causing proteins in other tumors.
“For the first time, cancer researchers now have the necessary tools to probe the molecular anatomy of tumor cells in search of cancer-causing proteins,” said Klausner. “Gleevec offers proof that molecular targeting works in treating cancer, provided that the target is correctly chosen. The challenge now is to find these targets.”