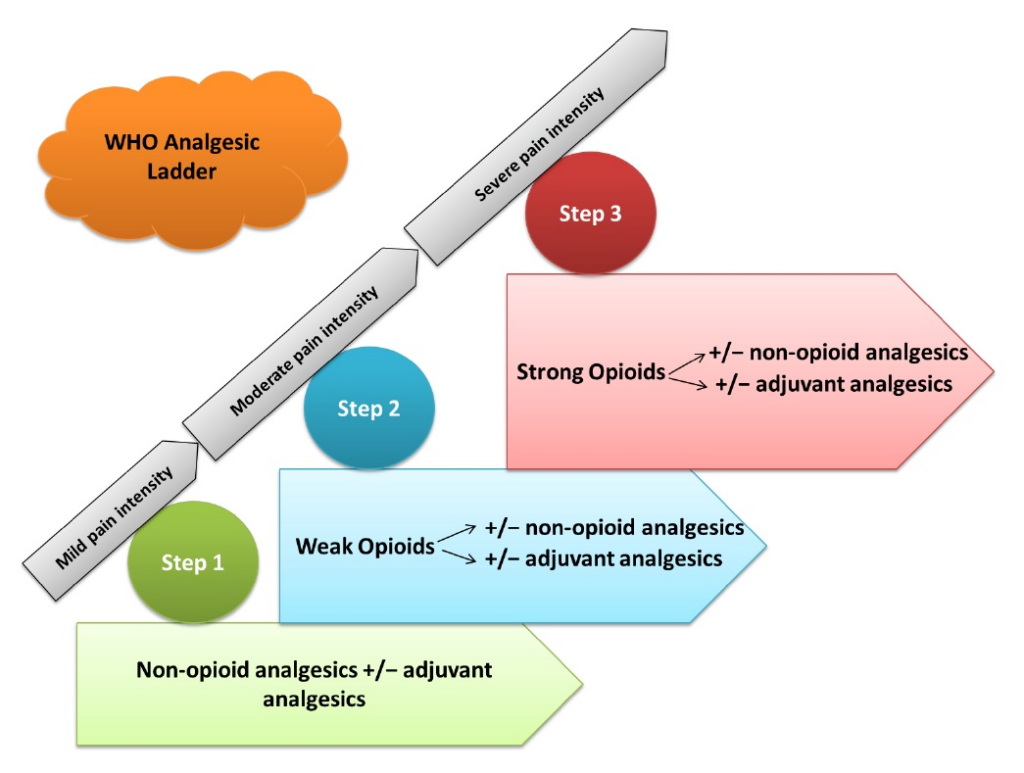
What are the strongest pain medications ?
Opioid analgesics, in general, are the strongest pain-relieving medications. The benchmark drug in this class is morphine — with other opioids falling above or below it in terms of pain-relieving potential. Near the bottom of the list is codeine, usually prescribed in combination with acetaminophen to relieve, for example, pain resulting from dental work. Codeine is only about 1/10th as powerful as morphine. Opioids more powerful than morphine include hydromorphone (Dilaudid) and oxymorphone (Opana). But the strongest opioid in community use is fentanyl which, in its intravenous form, is 70 to 100 times more potent than morphine. Fentanyl is also available as a long-release patch (Duragesic) and as a lozenge that dissolves in the mouth (Actiq). Sufentanil is even more powerful than fentanyl, but its use, at present is restricted to the intravenous route. However, a transdermal patch containing sufentanil is in clinical trials.
The term opioid refers to all compounds that bind to opiate receptors. Conventionally, the term opiate can be used to describe those opioids that are alkaloids, derived from the opium poppy; these include morphine and codeine. Opioids include semi-synthetic opiates, i.e., drugs that are synthesized from naturally occurring opiates (such as heroin from morphine and oxycodone from thebaine), as well as synthetic opioids such as methadone, fentanyl, and propoxyphene. The term narcotic is a legal designation and should not be used in the clinical setting; it refers to opioids and a few other drugs that are grouped with the opioids by law enforcement.
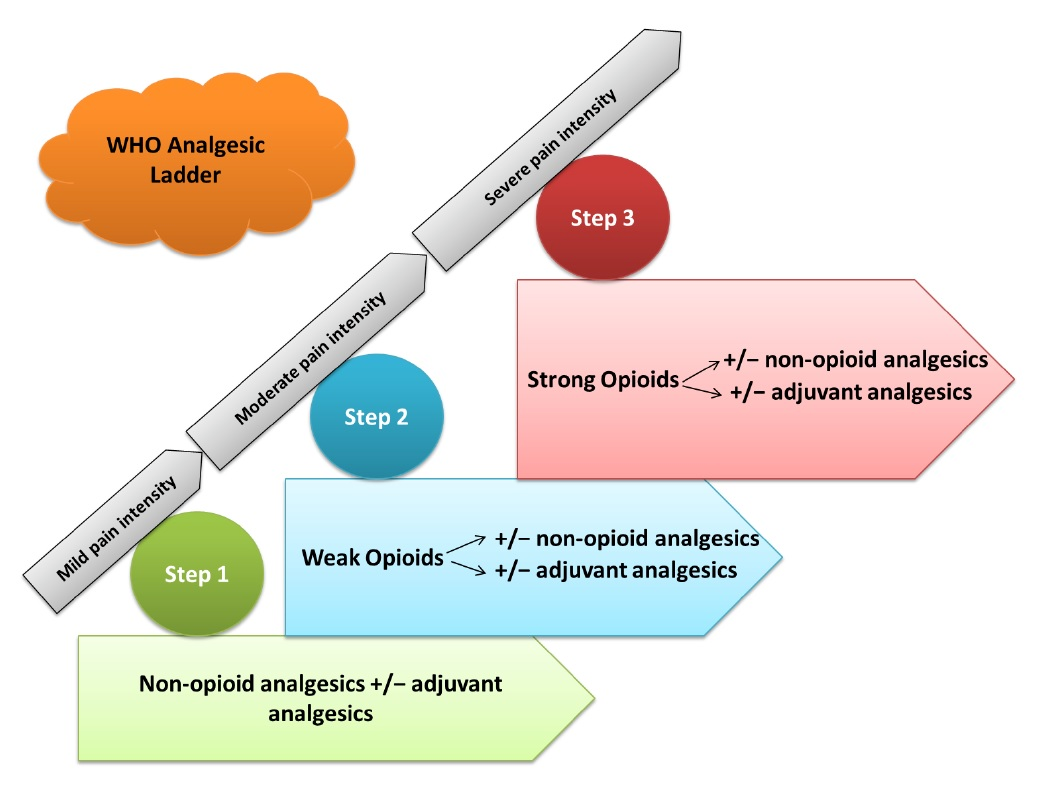
In the United States, numerous opioids have been commercialized for oral, transdermal and intravenous administration. Oral and transdermal formulations are usually administered for pain in the ambulatory setting. These include combination products, such as those containing hydrocodone and acetaminophen (Vicodin®, Lorset®) or ibuprofen (Vicoprofen®), tramadol and acetaminophen (Ultracet®), oxycodone and acetaminophen or aspirin (Percocet® or Percodan®), and those containing codeine and acetaminophen or aspirin. The single entity formulations on the market include those containing morphine (Avinza®, Kadian®, MS Contin®, MSIR®), oxycodone (OxyContin®), fentanyl (Duragesic®, Actiq®, Fentora®), hydromorphone (Dilaudid®), oxymorphone (Opana®), and methadone.
Opioids act by binding to specific proteins, called opioid receptors. Receptors are widely distributed. Those involved in pain modulation are situated in both the central nervous system and the peripheral nervous system. These receptors also bind endogenous opioid peptides (endorphins), which are involved in pain modulation and numerous other functions in the body. Among these functions are those mediated by deep structures of the brain, which are involved in the modulation of reinforcement and reward mechanisms, mood and stress. Opioid receptors are also found on cells from the immune system (Bidlack, 2000). In studies with rats, activation of these receptors with morphine is associated with varied effects, including sensitization of afferent nerves to noxious stimuli (Raghavendra, Rutkowski, & DeLeo, 2002).
When an opioid given for pain binds to receptors, analgesia may be accompanied by any of a diverse array of side effects related to the activation of receptors involved in other functions. These may include effects mediated by peripheral or by peripheral and central mechanisms, such as reduced peristalsis (leading to constipation) and itch, or primary central nervous system effects, such as miosis, (pupillary constriction) somnolence, mental clouding, and respiratory depression. Central mechanisms also lead to changes associated with hyperalgesia and decreased responsiveness to opioids (tolerance) and it has been speculated that opioid-induced hyperalgesia may be a clinically-relevant phenomenon leading to increased pain in some situations (Deleo, Tanga, & Tawfik, 2004). Activation of other central nervous system pathways by opioids also may produce mood effects, either dysphoria or euphoria.
Presumably, binding to those receptors involved in reinforcement and reward also occurs whenever an opioid is taken. In most individuals, when opioids are taken to treat pain, there appears to be no overt effect from change in these systems. In some cases, however, powerful reinforcement occurs, expressed as efforts to repeat the administration and these reinforcing outcomes may be associated with craving and with positive mood effects such as euphorigenic or pleasurable effects (Di Chiara, 2002; Koob & Bloom, 1988). These outcomes, which are uncommon but potentially serious when they occur (driving the development of an addictive pattern of use), can occur in the presence or absence of pain. Although these effects could be associated with iatrogenic addiction, they appear to be rare in patients who do not have risk factors suggesting the existence of the biological substrate for opioid-induced craving (see below).
Although several types of opioid receptors exist (e.g., mu, kappa and delta), opioid drugs largely produce their analgesic and reinforcing effects via activation of the mu opioid receptor; thus, opioids used for pain are often described as, “mu agonists”. Mu drugs that have the ability to fully activate opioid receptors (e.g., higher doses produce greater receptor activation in a dose-dependent manner) are referred to as opioid agonists or full mu agonists (such as morphine, oxycodone and methadone). Those opioids that occupy, but do not activate, receptors are referred to as opioid antagonists (e.g., naltrexone, naloxone); they can reverse the effects of mu opioid agonists. Those opioids that either have a low intrinsic activity at the mu receptor, or are agonists at another receptor and antagonists at the mu receptor are called agonist-antagonist drugs. Those with a low intrinsic activity are called partial opioid agonists and are characterized by a ceiling on most agonist activity, such that increases in dose will increase the drug’s physiological and subjective effects only to a certain level and further dose increases produce no additional effects (Jaffe & Martin, 1990).
These differences in mu receptor interactions are clearly related to the clinical use of opioid drugs and their abuse liability. Agonist-antagonist drugs are less attractive than pure mu agonists to individuals with addiction and no pain. Although other biochemical and molecular processes are presumably relevant to variation in these effects, relatively little is known about the interactions among these processes in humans.
The clinical use of opioid drugs is influenced by a variety of other characteristics, including pharmacokinetics. With the notable exception of methadone and buprenorphine, most opioids have relatively short half-lives and this has necessitated the development of new delivery systems designed to provide prolonged effects and a longer dosing interval.
Clinically-relevant physical dependence and tolerance (see below) may occur with short-term or long-term use of an opioid compound, particularly a pure mu agonist. These phenomena, which vary greatly in the clinical setting, represent neuroadaptational processes. The neurophysiology of physical dependence and tolerance are closely related to each other and to the phenomenon of opioid-induced hyperalgesia (Mao, 2002). The possibility that opioid administration, particularly at relatively high doses, may lead to increased pain has contributed to the controversy about opioid therapy for non-cancer pain, notwithstanding the limited evidence that this phenomenon occurs in clinical settings.
The benchmark drug in this class is morphine — with other opioids falling above or below it in terms of pain-relieving potential. Near the bottom of the list is codeine, usually prescribed in combination with acetaminophen to relieve, for example, pain resulting from dental work. Codeine is only about 1/10th as powerful as morphine.
Opioids more powerful than morphine include hydromorphone (Dilaudid) and oxymorphone (Opana). But the strongest opioid in community use is fentanyl which, in its intravenous form, is 70 to 100 times more potent than morphine.
Fentanyl is also available as a long-release patch (Duragesic) and as a lozenge that dissolves in the mouth (Actiq). Sufentanil is even more powerful than fentanyl, but its use, at present is restricted to the intravenous route. However, a transdermal patch containing sufentanil is in clinical trials.
Why opioids are dangerous?
When used as directed by your doctor, opioid medications safely help control acute pain, such as pain you experience after surgery. There are risks, though, when the medications are used incorrectly.
What opioid medications do
Opioids are a broad group of pain-relieving drugs that work by interacting with opioid receptors in your cells. Opioids can be made from the poppy plant — for example, morphine (Kadian, Ms Contin, others) — or synthesized in a laboratory — for example, fentanyl (Actiq, Duragesic, others).
When opioid medications travel through your blood and attach to opioid receptors in your brain cells, the cells release signals that muffle your perception of pain and boost your feelings of pleasure.
When opioid medications are dangerous
What makes opioid medications effective for treating pain can also make them dangerous.
At lower doses, opioids may make you feel sleepy, but higher doses can slow your breathing and heart rate, which can lead to death. And the feelings of pleasure that result from taking an opioid can make you want to continue experiencing those feelings, which may lead to addiction.
You can reduce your risk of dangerous side effects by following your doctor’s instructions carefully and taking your medication exactly as prescribed. Make sure your doctor knows all of the other medications and supplements you’re taking.