What are corticosteroids?
Corticosteroids are man-made drugs that closely resemble cortisol, a hormone that your adrenal glands produce naturally. Corticosteroids are often referred to by the shortened term “steroids.” Corticosteroids are different from the male hormone-related steroid compounds that some athletes abuse.
Corticosteroids are steroid hormones that are either produced by the body or are man-made.
Systemic corticosteroids refer to corticosteroids that are given orally or by injection and distribute throughout the body. It does not include corticosteroids used in the eyes, ears, or nose, on the skin or that are inhaled, although small amounts of these corticosteroids can be absorbed into the body.
Naturally occurring corticosteroids, hydrocortisone (Cortef) and cortisone, are produced by the outer portion of the adrenal gland known as the cortex (hence the name, corticosteroid). Corticosteroids are classified as either:
- glucocorticoids (anti-inflammatory) which suppress inflammation and immunity and assist in the breakdown of fats, carbohydrates, and proteins, or as
- mineralocorticoids (salt retaining) that regulate the balance of salt and water in the body.
Synthetic corticosteroids mimic the actions of naturally occurring corticosteroids and may be used to replace corticosteroids in people with adrenal glands that are unable to produce adequate amounts of corticosteroids, however, they more often are used in higher-than-replacement doses to treat diseases of immunity, inflammation or salt and water balance.
Examples of synthetic corticosteroids include:
- bethamethasone, (Celestone)
- prednisone (Prednisone Intensol)
- prednisolone (Orapred, Prelone)
- triamcinolone (Aristospan Intra-Articular, Aristospan Intralesional, Kenalog)
- methylprednisolone (Medrol, Depo-Medrol, Solu-Medrol)
- dexamethasone (Dexamethasone Intensol, DexPak 10 Day, DexPak 13 Day, DexPak 6 Day).
Some glucocorticoids also in addition to their anti-inflammatory actions have salt retaining properties but they are used mostly for their anti-inflammatory effects. Fludrocortisone (Florinef), a synthetic mineralocorticoid has strong salt retaining effects with significant anti-inflammatory actions, and is used mostly for it’s salt retaining capabilities.
What are some types of steroids?
Some corticosteroid medicines include cortisone, prednisone and methylprednisolone. Prednisone is the most commonly used type of steroid to treat certain rheumatologic diseases (like rheumatoid arthritis or lupus).
What are some examples of systemic (oral and injectable) corticosteroids?
The following is a list of the systemic (oral and injectable) corticosteroids that are available in the United States:
Glucocorticoids:
- hydrocortisone (Cortef)
- cortisone
- ethamethasoneb (Celestone)
- prednisone (Prednisone Intensol)
- prednisolone (Orapred, Prelone)
- triamcinolone (Aristospan Intra-Articular, Aristospan Intralesional, Kenalog) Methylprednisolone (Medrol, Depo-Medrol, Solu-Medrol)
- dexamethasone (Dexamethasone Intensol, DexPak 10 Day, DexPak 13 Day, DexPak 6 Day)
Mineralocorticoid:
- Fludrocortisone (Florinef)
How are steroids given?
Steroid medications are available in several forms that vary in how easily they dissolve or how long they stay in the body.
Steroids might be given locally, to the precise place where a problem exists, or systemically, which means throughout the “system” or body.
Examples of local steroid treatments include joint injections, eye drops, ear drops and skin creams. Systemic steroid treatments include oral medicines (given by mouth) or medicine that is delivered directly into a vein (intravenously or IV) or muscle (intramuscularly). Systemic steroids circulate through the bloodstream to various body sites.
When possible, local steroid treatments are prescribed instead of systemic steroids to reduce the risk of side effects.
How do steroids work?
Steroids work by decreasing inflammation and reducing the activity of the immune system. Inflammation is a process in which the body’s white blood cells and chemicals can protect against infection and foreign substances such as bacteria and viruses. In certain diseases, however, the body’s defense system (immune system) doesn’t function properly. This might cause inflammation to work against the body’s tissues and cause damage. Signs of inflammation include:
- Redness.
- Warmth.
- Swelling.
- Pain.
Steroids reduce the production of chemicals that cause inflammation. This helps keep tissue damage as low as possible. Steroids also reduce the activity of the immune system by affecting the way white blood cells work.
When are steroids given?
Steroids are used to treat many conditions in which the body’s defense system doesn’t work properly and causes tissue damage. Steroids may be the main therapy for certain diseases. For other conditions, steroids might only be used sparingly or when other measures have not been successful.
Steroids are used in the treatment for certain rheumatologic inflammatory conditions, such as:
- Systemic vasculitis (inflammation of blood vessels).
- Myositis (inflammation of muscle).
- Rheumatoid arthritis (chronic inflammatory arthritis).
- Systemic lupus erythematosus (a generalized disease caused by abnormal immune system function).
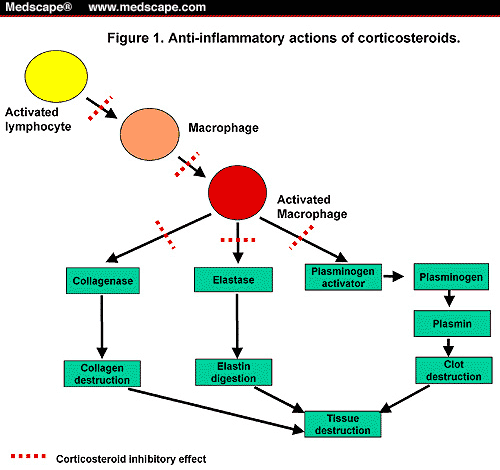
What are corticosteroids used for?
Corticosteroids belonging to the glucocorticoid class influence the body system in several ways, but they are used mostly for their strong anti-inflammatory effects and in conditions that are related to the immune system function such as:
- arthritis (for example, rheumatoid arthritis),
- colitis (ulcerative colitis, and Crohn’s disease),
- asthma,
- bronchitis,
- some situations involving skin rashes,
- allergic or inflammatory conditions involving the nose and eyes.
Glucocorticoid corticosteroids are used to treat systemic lupus, severe psoriasis, leukemia, lymphomas, idiopathic thrombocytopenic purpura, and autoimmune hemolytic anemia. These corticosteroids also are used to suppress the immune system and prevent rejection in people who have undergone organ transplant as well as many other conditions.
Fludrocortisone (Florinef), a potent systemic oral mineralocorticoid corticosteroid is used to treat Addison’s disease and diseases that cause salt loss as in congenital adrenal hyperplasia. It also is used commonly to treat conditions of low blood pressure (hypotension) although this is not a Food and Drug Administration (FDA) approved indication.
How are steroids beneficial?
When inflammation threatens to damage critical body organs, steroids can be organ-saving and in many instances, life-saving. For example, steroids may prevent the worsening of kidney inflammation, which could lead to kidney failure in people who have lupus or vasculitis. For these patients, steroid therapy might eliminate the need for kidney dialysis or transplantation.
Low doses of steroids might provide significant relief from pain and stiffness for people with rheumatoid arthritis. Short-term use of higher doses of steroids might help a person recover from a severe flare-up of arthritis.
What are the possible side effects of steroids and the adverse effects of steroids?
short-term and/or low-dose corticosteroid use results in few side effects. But taking corticosteroids long-term can result in severe side effects, including:
- Adrenal insufficiency — a condition in which the body cannot adequately respond to physical stress
- Atherosclerosis
- Bone death
- Cataracts and glaucoma
- Elevated blood pressure
- Elevated blood sugar
- Fluid retention
- Gastrointestinal bleeding
- Mood changes
- Osteoporosis
- Suppression of the immune system
- Trouble sleeping
- Weight gain
- Damage to local tissues
The chance of side effects depends on the dose, type of steroid and length of treatment. Some side effects are more serious than others. Common side effects of systemic steroids include:
- Increased appetite.
- Weight gain.
- Changes in mood.
- Muscle weakness.
- Blurred vision.
- Increased growth of body hair.
- Easy bruising.
- Lower resistance to infection.
- Swollen, “puffy” face.
- Acne.
- Osteoporosis (bone weakening disease).
- Onset of, or worsening of, diabetes.
- Onset of, or worsening of, high blood pressure.
- Stomach irritation.
- Nervousness, restlessness.
- Difficulty sleeping.
- Cataracts or glaucoma.
- Water retention, swelling.
These side effects are the most common side effects. All possible side effects are not included. Always contact your doctor if you have questions about your personal situation.
Factors influencing the adverse effects of glucocorticoids
Given the diversity in the mechanism of action of glucocorticoids, they can cause a wide array of adverse effects ranging from mild to severe, some of which are unavoidable. Of all the factors influencing the adverse effects of glucocorticoids, dose, and duration of therapy are the most important independent and well-documented risk factors. It is usually at “supra-physiologic” doses of corticosteroid administration where multiple and especially severe adverse effects of glucocorticoids occur, ranging from mild suppression of hypothalamic-pituitary axis to severe, life-threatening infections. However, long term use of low to moderate doses of glucocorticoids can lead to several serious adverse effects as well. Adverse effects of corticosteroids are both dose and time-dependent. Some adverse effects follow a linear dose-response pattern where the incidence increases with an increase in the dose (ecchymosis, cushingoid features, parchment-like skin, leg edema, and sleep disturbance). Other adverse effects may follow a threshold dose-response pattern with an elevated frequency of events beyond a specific threshold value (weight gain and epistaxis at prednisone dose greater than 5 mg daily, glaucoma, depression, hypertension at prednisone dose greater than 7.5 mg daily, etc.).
Several other factors may influence the adverse effects of glucocorticoids. Older age, comorbid conditions (such as diabetes mellitus), concomitant use of other immunosuppressive agents, severity, and nature of the underlying disease and poor nutritional status can all influence the occurrence and magnitude of side-effects.
Musculoskeletal adverse effects
Glucocorticoids induced Osteoporosis is one of the well-known and devastating adverse effects of long-term use of glucocorticoids. Up to 40% of patients on long-term glucocorticoids develop bone loss leading to fractures. Several mechanisms play a role, including osteoclast activation by promoting RANK-ligand as well as a decrease in function and number of osteoblasts and osteocytes. The trabecular bone is initially affected, with cortical bone loss seen with longer-term use. The loss of trabecular bone can occur within the first 6 to 12 months of therapy.
Steroid-induced myopathy, which is a reversible painless myopathy and is a direct result of muscle breakdown, can occur in both the upper and lower extremities, usually with high-dose long-term use of glucocorticoids. Muscle enzymes (CK and Aldolase) are typically normal, and findings on electromyography are non-specific. Muscle biopsy reveals Type-II fiber atrophy without inflammation. Withdrawal of glucocorticoids and exercises usually results in resolution of the myopathy. “Critical illness myopathy” may also develop in patients admitted in the intensive care unit (ICU) requiring large doses of IV glucocorticoids and neuromuscular blocking agents. It characteristically presents with a severe, diffuse, proximal, and distal weakness that develops over several days. Although it is usually reversible, critical illness myopathy can lead to prolonged ICU admissions, increased length of hospital stays, severe necrotizing myopathy, and increased mortality.
Osteonecrosis can be seen especially with long-term use of prednisone more than 20 mg daily. Patients with SLE and children are at higher risk. Hips and knees are the most commonly involved joints with less common involvement of shoulders and ankles. Pain is the initial feature, which may eventually become severe and debilitating. Magnetic resonance imaging is the most sensitive test, especially for early detection. Plain radiographs may be negative initially but can be useful for follow-up. Treatment is by decreased weight-bearing and immobilization initially, but if severe, surgery and/or joint replacement may be necessary.
Metabolic and endocrine adverse effects
Systemic glucocorticoids cause a dose-dependent increase in fasting glucose levels and a more significant increase in postprandial values in patients without preexisting diabetes mellitus, but the development of de novo diabetes in a patient with initially normal glucose tolerance is uncommon. Risk factors for new-onset hyperglycemia during glucocorticoid therapy appear to be the same as those for other patients. However, patients with diabetes mellitus or glucose intolerance exhibit higher blood glucose levels while taking glucocorticoids, leading to increased difficulty with glycemic control.
The development of cushingoid features (redistribution of body fat with truncal obesity, buffalo hump, and moon face) and weight gain are dose and duration-dependent and can develop early. Cushingoid features showed a linear increase in frequency with dosing. Glucocorticoid therapy is the most common cause of Cushing syndrome. The clinical presentation in the pediatric population is similar to that in adults and includes truncal obesity, skin changes, and hypertension. In children, growth deceleration is also a feature.
Administration of glucocorticoids can suppress the hypothalamic-pituitary-adrenal (HPA) axis decreasing corticotropin-releasing hormone (CRH) from the hypothalamus, adrenocorticotropic hormone (ACTH) from the anterior pituitary gland and endogenous cortisol. Prolonged ACTH suppression cause atrophy of adrenal glands, and abrupt cessation or rapid withdrawal of Glucocorticoids in such patients may cause symptoms of adrenal insufficiency. The clinical presentation of adrenal suppression is variable. Many of the signs and symptoms are non-specific and can be mistaken for symptoms of intercurrent illness or the underlying condition that is receiving treatment (weakness/fatigue, malaise, nausea, vomiting, diarrhea, abdominal pain, headache usually in the morning, fever, anorexia/weight loss, myalgia, arthralgia, psychiatric symptoms, poor growth and weight gain in children). Adrenal suppression is the most common cause of adrenal insufficiency in children and is associated with higher mortality in the pediatric population. In adults, the symptoms of adrenal suppression are non-specific; therefore, the condition may go unrecognized until exposure to physiological stress (illness, surgery, or injury), resulting in an adrenal crisis. Children with adrenal crisis secondary to adrenal suppression may present with hypotension, shock, decreased consciousness, lethargy, unexplained hypoglycemia, seizures, and even death.
The impairment of growth in young children and delay in puberty commonly presents in children receiving glucocorticoids for chronic illnesses like nephrotic syndrome and asthma. The effect is most pronounced with daily therapy, and less marked with an alternate-day regimen and can also occur with inhaled glucocorticoids. Although growth impairment can be an independent adverse effect of corticosteroid therapy, it can also be a sign of adrenal suppression.
Infections
Moderate to high dose use of glucocorticoids poses a significant risk of infections, including common mild infections as well as serious life-threatening infections. There is a linear increase in the risk with dose and duration of therapy, especially with common bacterial, viral, and fungal pathogens. Concomitant use of other immunosuppressive agents and the elderly age further increases the risk of infections. Prednisone dose of less than 10 mg daily pose minimal to no risk of infection. Patients taking glucocorticoids may not manifest common signs and symptoms of infection as clearly, due to the inhibition of cytokine release and the associated reduction in inflammatory and febrile responses leading to a failure in early recognition of infection.
Cardiovascular adverse effects
Mineralocorticoid effects, especially as seen with cortisol and cortisone, can lead to fluid retention, edema, weight gain, hypertension, and arrhythmias by increasing renal excretion of potassium, calcium, and phosphate. Hypertension usually occurs with higher doses only. Long-term use of medium-high dose glucocorticoids has implications in premature atherosclerosis in a dose-dependent pattern.
Dermatologic adverse effects
Several cutaneous adverse effects can occur even at a low dose use of glucocorticoids, although the risk increases linearly with the increasing dose and duration of glucocorticoid therapy. Although cutaneous adverse effects appear to be clinically significant by physicians, they are usually of most concern to the patients. These adverse effects include ecchymosis, skin thinning and atrophy, acne, mild hirsutism, facial erythema, stria, impaired wound healing, thinning of hair, and perioral dermatitis.
Ophthalmologic adverse effects
The risk of cataract is significantly high in patients taking prednisone more than 10 mg daily for more than one year, with a dose-dependence in a linear fashion. However, an increased risk of cataracts has been reported even with low-dose glucocorticoids.[14] Cataracts are usually bilateral and slowly progressing. Increased intraocular pressure, especially in patients with a family history of open-angle glaucoma, is seen in patients receiving intraocular glucocorticoids, and high dose systemic glucocorticoids. Glaucoma is often painless and can lead to visual field loss, optic disc cupping, and optic nerve atrophy. After the discontinuation of systemic therapy, the elevation in intraocular pressure usually resolves within a few weeks, but the damage to the optic nerve is often permanent. A rare adverse effect of systemic or even topical use of glucocorticoids is central serous chorioretinopathy; this leads to the formation of subretinal fluid in the macular region, which leads to separation of the retina from its underlying photoreceptors. This condition manifests as central visual blur and reduced visual acuity.
Gastrointestinal (GI) adverse effects
Glucocorticoids increase the risk of adverse GI effects, such as gastritis, gastric ulcer formation, and GI bleeding. The use of NSAIDs and glucocorticoids is associated with a 4-fold increased risk of a GI adverse effect compared with the use of either drug alone. Other complications associated with glucocorticoid use include pancreatitis, visceral perforation, and hepatic steatosis (fatty liver) that can rarely lead to systemic fat embolism or cirrhosis.
Neuropsychiatric adverse effects
Patients receiving glucocorticoids often experience an improved sense of well-being within several days of starting the medications; mild euphoria or anxiety may also occur. Hypomanic reactions and activated states are more common early in the therapy than depression, but the prevalence of depression is greater in patients on more longstanding therapy. Psychosis can occur but does so almost exclusively at doses of prednisone above 20 mg per day given for a prolonged period. Disturbances in sleep are reported, especially with split doses that may interfere with the normal pattern of diurnal cortisol production. Akathisia (motor restlessness) is a common glucocorticoid side effect. The risk of developing a given neuropsychiatric disorder following glucocorticoid therapy may increase among patients with a history of the condition. Rare cases of pseudotumor cerebri have also correlated with glucocorticoid use.
Does everyone have side effects?
Not all patients will develop side effects. How often any side effect occurs varies from person to person.
If steroid use is brief (from a few days to a few weeks), it is possible that none of the listed side effects will occur. The side effects listed here generally do not occur when occasional steroid injections are given for arthritis, tendonitis or bursitis. However, if steroid use involves high doses and is prolonged (for a few months to several years), an increase in the number of side effects might occur. The prolonged use of high dose steroids is justified only for severe illnesses that represent serious risks to the patient.
How can the side effects of steroids be minimized?
To minimize the side effects of steroids, healthcare providers follow several guidelines:
- Use steroids only when necessary.
- Watch the patient closely to detect early signs of serious side effects.
- If possible, use local steroids for local problems.
- Use the smallest dose needed to control the disease.
- Reduce the dose gradually as long as the disease remains under control.
- Monitor blood pressure and blood sugar often and treat if necessary.
- Monitor bone density and prescribe medications and supplements to help bone health.
There are other ways to prevent certain side effects, and these need to be discussed individually with your healthcare provider.
What are the differences between the types of systemic corticosteroids?
Corticosteroids differ in their relative amount of anti-inflammatory and mineralocorticoid potency and they are used according to these effects. Among the systemic (oral and injectable) corticosteroids, fludrocortisone (Florinef) has the most significant mineralocorticoid (salt retaining) actions and is best used for this effect despite it’s strong anti-inflammatory action.
Other systemically available corticosteroids have mostly glucocorticoid effects, and are used for their anti-inflammatory activities. Examples of these include the naturally occurring hydrocortisone (Cortef) and cortisone, and the synthetic corticosteroids including:
- bethamethasone (Celestone)
- prednisone (Prednisone Intensol)
- prednisolone (Orapred, Prelone)
- triamcinolone (Aristospan Intra-Articular, Aristospan Intralesional, Kenalog)
- methylprednisolone((Medrol, Depo-Medrol, Solu-Medrol)
- dexamethasone (Dexamethasone Intensol, DexPak 10 Day, DexPak 13 Day, DexPak 6 Day).
Among all glucocorticoids, prednisone is not effective in the body unless it is converted to prednisolone by enzymes in the liver. For this reason prednisone may not be very effective in people with liver disease because of a reduction in their ability to convert prednisone to prednisolone.
What drugs interact (contraindications) with corticosteroids?
Certain drugs such as troleandomycin (TAO), erythromycin (Ery-Tab, EryPed 200), and clarithromycin (Biaxin) and ketoconazole (Nizoral) can reduce the ability of the liver to metabolize (breakdown) corticosteroids and this may lead to an increase in the levels and side effects of corticosteroids in the body. On the other hand, phenobarbital, ephedrine, phenytoin (Dilantin), and rifampin (Rifadin, Rimactane) may reduce the blood levels of corticosteroids by increasing the breakdown of corticosteroids by the liver. This may necessitate an increase of corticosteroid dose when they are used in combination with these drugs.
Estrogens have been shown to increase the effects of corticosteroids possibly by decreasing their breakdown by the liver.
Corticosteroid effects on warfarin (Coumadin) can vary; therefore when taking warfarin (Coumadin) along with corticosteroids, there may be increased need for monitoring coagulation levels more closely.
Low blood potassium (hypokalemia) and a higher chance of heart failure can result from combining corticosteroids with drugs that reduce potassium in the blood (for example, diuretics, amphotericin B).
Anticholinesterase drugs (for example, physostigmine) may cause severe weakness in some patients with myasthenia gravis when prescribed with corticosteroids.
Corticosteroids can increase blood glucose, so close monitoring of blood sugar and higher doses of diabetes medications may be needed.
Cholestyramine (Questran, Questran Light) can decrease the absorption of oral corticosteroids from the stomach and this could reduce the blood levels of corticosteroids.
Reference:
ncbi.nlm.nih.gov/books/NBK531462/